- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
- White Papers
Demystify New Regulations for Hazardous Waste

After many years of deliberation, the Environmental Protection Agency (EPA) published its final rule on pharmaceutical waste management on February 22, 2019: Management Standards for Hazardous Waste Pharmaceuticals and Amendment to the P075 Listing for Nicotine, also known as “Subpart P.” While the ruling became effective on August 21, 2019 in states and territories that are managed by EPA, all other states must actively adopt the stricter portions of it within one to two years, whether by regulation or legislation. As of this writing, 23 states operate under the new rule (see FIGURE 1).1 Before delving into the benefits of Subpart P, we will review the difference between a hazardous drug and a hazardous waste, as there continues to be confusion between these two definitions.
Hazardous Waste Defined
Hazardous waste was defined by the EPA in 1976 via the Resource Conservation and Recovery Act (RCRA). In this regulation hazardous waste is identified by four characteristics: toxicity, ignitability, corrosivity, and reactivity. Additionally, certain highly toxic chemicals were listed as inherently hazardous when they were the sole active ingredient in the waste. These P- and U- listed chemicals include a number of common drugs found in practice settings (see TABLE 1).

Various drugs meet the toxicity and ignitability definitions, including multi-dose flu vaccine, insulin vials, and paclitaxel before dilution. These are identified with either the code D001 for ignitability or a specific D code for toxicity. Approximately 5% of drugs on the market are designated as hazardous waste when discarded. For a more detailed discussion of these definitions, please refer to Managing Pharmaceutical Waste, A Ten-Step Blueprint for Healthcare Facilities in the United States.2
No new drugs have been added to the list since its creation, and EPA has responded to a 2012 Inspector General report expressing concerns in this regard by referring to the benefits offered in Subpart P for healthcare facilities to manage all waste pharmaceuticals as hazardous waste pharmaceuticals.3 A cost analysis to determine the increased disposal costs inherent in this approach was not provided. The pharmacy profession most often manages highly toxic antineoplastic agents as hazardous waste as a best practice to close this environmental gap.
NIOSH Hazardous Drug Definition
In contrast to the static hazardous waste P- and U- list, the NIOSH hazardous drug list continues to evolve. The primary criteria for drugs on this list are: genotoxicity, teratogenicity, reproductive toxicity, carcinogenicity, organ toxicity at low doses, and similar structure or toxicity profile. Although the draft NIOSH List of Hazardous Drugs in Healthcare Settings, 2020 only includes drugs approved through 2015, it is more up-to-date than the EPA’s P- and U- lists which remain the same as in 1976.
It is important to remember when communicating specific management policies and procedures to pharmacy and nursing, that hazardous drugs must be managed in a way that primarily protects people, while hazardous waste disposal practices serve to protect the environment. As is evident in FIGURE 2, only a few drugs meet the criteria for both definitions. Messaging to pharmacy, nursing, and other healthcare practitioners identifying hazardous drugs must be clear and separate from messaging identifying hazardous waste.

Benefits of the New Hazardous Waste Rules
One of the key benefits of the new ruling is the exemption of OTC nicotine lozenges, gums, and patches from the P-list. This is separate from Subpart P, and because it is less strict, states are not required to adopt it. However, some states have adopted this exemption prior to adopting the full regulation and most are expected to follow suit. If you are in a state that has adopted this exemption, you will no longer need to manage nicotine wrappers as hazardous waste and can instead dispose of them in the trash. However, used and unused patches should be disposed of as non-hazardous pharmaceutical waste due to the concentration of nicotine remaining in them. Note that prescription nicotine products are still regulated, as are vaping products.
Additionally, Subpart P exempts empty warfarin wrappers from hazardous waste by changing the requirements for empty packages. Under the new regulation, stock or dispensing bottles of 1 liter, 10,000 tablets or capsules or less, and unit dose containers are considered empty if all the contents have been removed. IV bags are considered empty when fully administered, most of which can be disposed of in the trash. However, empty chemotherapy containers, including IV bags, should be managed in the yellow trace chemotherapy containers: while not a federal requirement, this is a best practice, mandated in several states, and recommended under USP <800>. Syringes are considered empty when fully depressed. Several dosage forms are not considered to ever be empty, including inhalers, aerosols, nebulizers, creams, gels, and ointments.
Impact on Generator Status
Since hazardous waste pharmaceuticals are often the largest contributors to the amount of hazardous waste generated per month, another major benefit realized from these rules is a potential reduction in generator status. This may result in health care facilities moving from large quantity generator (LQG) status to small (SQG) or even very small quantity generator (VSQG) status. To evaluate whether this may apply to your facility, see TABLE 2.

State Registration
If your facility is currently an LQG or SQG prior to Subpart P, your facility needs to register with the state by completing Form 8700-12 within 60 days of when Subpart P becomes effective, or with your next annual or biennial report, depending on the state. This process is usually the responsibility of your Environmental Services Manager or Facility Manager; however, they may not be aware of this requirement. For more information on this process, refer to TABLE 3.

Labeling Requirements
Under Subpart P, the only label needed on a hazardous waste container is “Hazardous Waste Pharmaceuticals.” Facilities must, however, ship said container within one year of the start date as well as have a system for tracking the residence time of the container. The simplest method to fulfill this requirement is to place the start date on the label of the container when it is placed into service. This eliminates the concept of “satellite accumulation” that has been previously used for the placement of hazardous pharmaceutical waste containers. However, containers must still be kept closed when not in active use. Your hazardous waste vendor will provide the shipping label and the manifest. Instead of listing all the waste codes, the term PHRM is now required in box 13 on the manifest. Again, this part of the process is usually performed by the Environmental Services Manager, but it is important to ensure they are familiar with these changes.
To Sort or Not to Sort
The EPA encourages all waste pharmaceuticals to be managed in an environmentally safe manner, including avoiding sewering of any drugs. One option is to manage all pharmaceutical waste as hazardous waste, which eliminates the need to identify the hazardous waste codes or segregate any drug waste other than incompatibles, such as pressurized aerosols, acids, bases, oxidizers, and the heavy metal arsenic trioxide. While this simplifies training and containment strategies, it also significantly increases the cost, since the majority of drugs become non-hazardous waste if discarded. The cost-benefit ratio will need to be examined by each organization to determine what makes the most sense in terms of implementation costs versus dollars saved. Managing all drugs, except IV salts which can still be drain disposed, by sorting into hazardous or non-hazardous waste is another way to accomplish EPA’s goals, at a reduced cost to the organization.
Controlled Substance Disposal
Under Subpart P, there are several drugs that are both DEA controlled substances and EPA hazardous wastes, such as injectable diazepam, chloral hydrate, testosterone gel, and fentanyl sublingual spray, and are either listed or characteristic hazardous wastes. Subpart P exempts these drugs from disposal as hazardous waste if they meet either of two qualifications: they are destroyed in a manner publicly deemed in writing by DEA to render them non-recoverable, or they are incinerated in an appropriate type of incinerator. Qualified incinerators are either: a large or small municipal waste combustor; a hospital, medical, and infectious waste incinerator; a commercial and industrial solid waste incinerator; or a hazardous waste combustor. As the DEA has not publicly approved in writing any method of wastage other than incineration or chemical digestion, ensuring that any controlled substance wastage is incinerated enables an organization to take advantage of this exemption. This leads back to the DEA Drug Disposal Regulations and the differentiation between inventory and wastage.
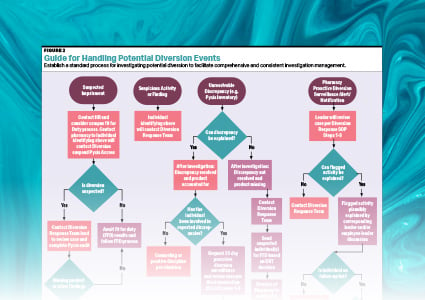
Based on numerous statements from DEA representatives, if an outdated or unwanted controlled substance is still in the pharmacy’s inventory (ie, has not been dispensed), it must either be sent to a reverse distributor as a transfer between registrants or two employees must witness its destruction by incineration and complete a Form 41. Since many health care facilities do not have ready access to incinerators, reverse distribution is a practical alternative. Once a controlled substance has been dispensed to a patient, any drug that has not been administered, such as a partial morphine IV, is considered wastage by the DEA. At this point, the only DEA requirement is to document the wastage and to dispose of it in a manner that prevents diversion and meets all local, state, federal, and tribal environmental regulations. If the controlled substance is also a hazardous waste, it cannot be drain disposed. To prevent diversion, the use of a sequestration device is recommended. The device or cartridge can then be discarded into the non-hazardous pharmaceutical waste container bound for incineration. If the facility stocks any of the controlled substances that meet the hazardous waste definition, serious consideration should be given to alternatives to drain disposal throughout the facility, since it can be difficult to institute effective training for the disposing of one controlled substance into a sequestration device and others down the drain (see DIAGRAM).
Hazardous Waste Regulations and USP <800>
When constructing policies and procedures to meet both hazardous drug and hazardous waste compliance requirements, it is important to note that EPA does not specifically address any wastage unless it is either a regulated hazardous waste or overtly contaminated with hazardous waste. In Subpart P, EPA states: “…the contained in policy would not apply to gloves that have touched a warfarin pill during the course of patient care. However, if a healthcare worker spills a hazardous waste pharmaceutical on their personal protective equipment and it cannot be removed …the personal protective equipment would be considered a hazardous waste pharmaceutical.”4 This means that the disposal of many chemotherapy drugs and most NIOSH hazardous drugs are not addressed by the EPA hazardous waste regulations. While some states do regulate these drugs and trace chemotherapy items, we will examine the interface between USP <800>, NIOSH hazardous drugs, and EPA hazardous waste only at the federal level.
Disposal Messaging
Many health care organizations have developed creative messages for identifying drugs that become hazardous waste. In the pharmacy, the most common method is the old-fashioned shelf sticker, using codes such as P, U, or D. Some facilities now under Subpart P simply use a black sticker for most hazardous waste pharmaceuticals to indicate disposal in the black container. With respect to nursing, messaging should occur in the automated dispensing cabinet, the medication administration record, and on the label when possible, such as in the case of IVs and creams, ointments, inhalers, etc. Under Subpart P, warfarin and nicotine wrappers are no longer classified as hazardous waste, which not only simplifies the process but adds to credibility. If the facility is using only hazardous waste containers, then little or no messaging is needed, other than to ensure that incompatible drugs, such as aerosols and oxidizers, are segregated, biohazardous items are disposed into the red container, and all other drugs are placed into the black container.
As noted earlier, there is an overlap between the EPA’s designation of hazardous waste pharmaceuticals and NIOSH hazardous drugs. While these encompass two very different definitions, the use of the common term “hazardous” has led to a great deal of confusion. Everyone in your organization who may encounter these products needs to clearly understand the differences and how it applies to them. It is crucial that your messaging and policies and procedures overtly address these differences.
Messaging for HD Waste Management
When determining appropriate messaging for USP <800> hazardous drug waste management there are several distinct steps. Any hazardous drugs that are also on the P- and U- list should be managed as hazardous waste. Likewise, the formulation should be examined to determine if the drug meets any of the characteristics of hazardous waste, primarily ignitability or toxicity. In addition, your organization should check your state regulations to determine if additional drugs are listed as hazardous waste or if “bulk chemotherapy” is called out for hazardous waste management. Finally, your organization should consider implementing best practices of managing antineoplastic hazardous drugs as hazardous waste. The messaging for hazardous waste management should be separate and distinct from the messaging for hazardous drug identification.
Disposal of Personal Protective Equipment
One of the more challenging decisions to make involves the management and disposal of personal protective equipment (PPE). Concerning hazardous drugs, Table One of the currently accepted NIOSH list makes a simple differentiation regarding PPE used in the preparation and administration of antineoplastic drugs. Although these items are required to be managed as trace chemotherapy and incinerated at a regulated medical waste facility in several states, their management as such is also generally a matter of good professional practice standards. USP <800> has been rather vague in this regard, referring to management as “hazardous waste” in a generic sense only.
To operationalize this guidance, consider disposing of all trace-contaminated chemotherapy PPE waste generated in the pharmacy and nursing units in the yellow trace chemotherapy container or hamper. PPE used in handling other hazardous drugs can be placed in trash unless overtly contaminated with hazardous drugs. In the case of cleaning up a hazardous drug spill, the PPE should be managed as hazardous waste.
Conclusion
The introduction of the EPA’s Subpart P regulation provides a more pragmatic approach to the management of hazardous waste pharmaceuticals and their packaging. Since most states must actively adopt Subpart P, it will be important for pharmacy managers to ascertain which regulation applies in their state. Also, pharmacists should be considering guidance established by USP <800> and the NIOSH list of hazardous drugs and the differentiation from hazardous waste pharmaceuticals. Drug disposal guidance versus PPE requirements must be coded separately on IV labels, in the automated dispensing cabinets, and the medication administration records. Ideally, once established, these systems will require only minor adjustments as new drugs enter the market.
References
- US Environmental Protection Agency. Where are the Management Standards for Hazardous Waste Pharmaceuticals and Amendment to the P075 Listing for Nicotine in Effect? Updated April 5, 2021. Accessed April 19, 2021. https://www.epa.gov/hwgenerators/where-are-management-standards-hazardous-waste-pharmaceuticals-and-amendment-p075.
- Pines E, Smith C. Managing Pharmaceutical Waste: A 10-Step Blueprint for Healthcare Facilities in the United States. Practice Greenhealth. Published April 15, 2006. Revised August 2008. Accessed April 19, 2021. https://practicegreenhealth.org/sites/default/files/upload-files/pharmwasteblueprint.pdf.
- Lewis E, Crawford D, Chang M, Manning N. Follow-Up Report: EPA Proposes to Streamline the Review, Management and Disposal of Hazardous Waste Pharmaceuticals. Report No. 15-P-0260. US Environmental Protection Agency. Published August 19, 2015. Accessed April 19, 2021. https://www.epa.gov/sites/production/files/2015-09/documents/20150819-15-p-0260.pdf.
- US Environmental Protection Agency. Management Standards for Hazardous Waste Pharmaceuticals and Amendment for the P075 Listing for Nicotine. Fed Regist. 2019;84(36):5816-5950.
 Charlotte Smith, RPh, MS is Senior Regulatory Advisor, PharmEcology Services, a business unit of WM Sustainability Services. She is a registered pharmacist who received her BS in pharmacy and MS in continuing and vocational education from the University of Wisconsin. Charlotte founded PharmEcology Associates, LLC in 2000 and sold the company to Waste Management in 2009. She co-founded Capital Returns, Inc, a nationally known pharmaceutical reverse distributor in 1991 and for 10 years served as president and chief regulatory advisor. Charlotte is a member of the Pharmacy Society of Wisconsin, the American Society of Health-System Pharmacists, and the American Society of Consultant Pharmacists.
Charlotte Smith, RPh, MS is Senior Regulatory Advisor, PharmEcology Services, a business unit of WM Sustainability Services. She is a registered pharmacist who received her BS in pharmacy and MS in continuing and vocational education from the University of Wisconsin. Charlotte founded PharmEcology Associates, LLC in 2000 and sold the company to Waste Management in 2009. She co-founded Capital Returns, Inc, a nationally known pharmaceutical reverse distributor in 1991 and for 10 years served as president and chief regulatory advisor. Charlotte is a member of the Pharmacy Society of Wisconsin, the American Society of Health-System Pharmacists, and the American Society of Consultant Pharmacists.
Kathleen Skibinski, RPh, MS is Manager of Regulatory and Compliance for PharmEcology Services, a business unit of WM Sustainability Services. She received her BS in pharmacy and MS in hospital pharmacy administration from the University of Wisconsin – Madison. Kathy is a member of the Pharmacy Society of Wisconsin and the American Society of Health-System Pharmacists.
Monica Livingston is Implementation Manager for PharmEcology, a business unit of WM Sustainability Services. She co-founded Capital Returns, Inc, a pharmaceutical reverse distributor, in 1991 and served as VP of Operations through 1999. Monica has been instrumental in developing the entire PharmEcology Services program almost since its inception in 2000. She is responsible for managing this business unit for Waste Management and delivering all on-site consulting and training services.
Like what you've read? Please log in or create a free account to enjoy more of what www.pppmag.com has to offer.