- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
- White Papers
Weighing Unit Dose Repackaging Options
With US hospitals rapidly adopting bar code medication administration (BCMA), the need for unit dose packaging has grown dramatically in order to ensure 100% bar code compliance for scanning on administration. According to the most recent State of Pharmacy Automation Survey (PP&P, August 2012), 59% of US hospitals have adopted BCMA as of 2012 and 80% of remaining hospitals plan to adopt it in the near future, more than three-quarters of them within two years. Further fueling the need to repackage is the increasing trend of drug manufacturers supplying medication in bulk packaging rather than unit dose to cut costs. Pharmacy departments are therefore faced with three medication repackaging options: creating an in-house repackaging center, outsourcing to a medication repackaging vendor, or a mixture of both. All three methods offer advantages and disadvantages that must be considered before choosing the best option for your facility.
The Medical University of South Carolina Medical Center (MUSC) is a 700-bed academic medical center located in Charleston, South Carolina. The medical center adopted BCMA in 2006, and initially chose to implement an in-house medication repackaging center, investing in staff, repackaging equipment, supplies, and facilities to house the operation. Our repackaging center has a dedicated staff (one FTE pharmacist and two FTE technicians) that operates a large bulk repackager and a tabletop repackager, as well as an overwrap machine to support repackaging needs for an inpatient robot, medication carousels, and automated dispensing cabinets, along with our organization-wide BCMA system. The choice for in-house repackaging at that time was made for a variety of reasons, including shorter turnaround time, ability to manage inventory levels, financial support from hospital administration, and our lack of knowledge regarding outsourcing repackaging options.
Reconsidering Our Repackaging Model
We recently began to reconsider our initial model. In reviewing the net expense of running a large-scale in-house repackaging operation for 1.4 million doses annually—including the combined costs for equipment, maintenance, and supplies, as well as various ancillary costs—we found our expenditures to be higher than we anticipated. The ancillary costs comprised the staff operating the repackaging equipment, as well as the pharmacist conducting quality checks and completing the documentation for each batch. In addition, due to growth at our institution, we were experiencing increasing workloads in other critical pharmacy areas (eg, compounding and controlled substances), but were unable to hire new staff, which made it clear that a staffing shift was necessary to manage the additional workload.
Recognizing these limitations, we decided to evaluate the financial impact of moving the majority of our repackaging to an outsourced vendor. We assessed a variety of factors, starting with cost. Noel Hodges, PharmD, MBA, provided a helpful algorithm for gauging unit dose costs in a PP&P article (“To Package or Not to Package,” March 2007; see algorithm online at pppmag.com/algo), which laid out the metrics to determine the best path for unit dose medication purchasing based on the cost per repackaged dose. To calculate in-house per-unit cost, for example, it is important to factor in the cost of labor, equipment, service and maintenance agreements, regulatory documentation, supplies, etc. Dividing that total by the number of doses repackaged provides a clear basis to compare your per-unit costs against those of an outsourced repackager. Currently, we estimate our per-dose cost for in-house repackaging for oral solids to be about $0.25. As we consider this cost to be high, it justified our search for other options. We encourage other organizations to conduct this same analysis as a way to lower costs.
Of course, there are a variety of other considerations in addition to cost. Given the risk of potentially exposing your staff and equipment to hazardous contamination, for example, outsourcing drugs such as oral chemotherapy to vendors better equipped for such processes may be the most prudent approach. Other options include outsourcing controlled substances to minimize liability, or outsourcing oral solutions given the labor required to draw up large quantities of product. Conversely, unit dose packaging of small batches of infrequently used medications might be more efficiently processed in-house. Turnaround time is another point to consider; what can be done same-day in-house may take up to 72 hours from an outsourced repackager, although a constant focus on timely ordering and inventory monitoring will minimize this issue. Therefore, institutions may want to consider a hybrid model that includes both outsourcing and in-house options for same-day needs.
Choosing a Repackaging Vendor
Should you choose to outsource your repackaging needs, there are many points to keep in mind when selecting a vendor. (See Table 1.) Price is a consideration, of course, but the recent contamination of outsourced sterile compounding products is a reminder that it is vital to perform due diligence with the vendor, including a site visit to the facility. The vendor should be compliant with FDA guidance, exceed the appropriate state board of pharmacy requirements, and be in compliance with all applicable regulations and guidelines, including USP. Check the vendor’s history: determine how long they have been in business, call references, and review any past regulatory citations. The vendor should be able to clearly demonstrate their repackaging processes through standard operating procedure documents; be alert for any possible gaps in procedures. Confirm that the vendor can meet your facility’s labeling requirements. You also want to review the ordering process, turnaround time from receipt of the bulk medication to arrival at your pharmacy, and processes for handling recalls. While this may appear to be an intensive process for selecting a vendor, keep in mind that, even though the vendor is repackaging another supplier’s medications, you are responsible for ensuring the integrity of the vendor’s site and any product entering your institution.
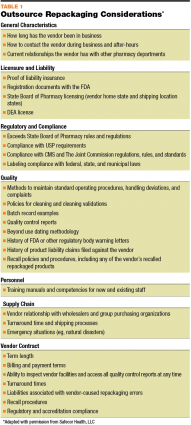
Click here to view a larger version of this Table
In the transition to an outsourced repackaging vendor, it makes sense to start with high-volume, fast-moving products. Once those processes are established to your satisfaction, consider expanding your outsourcing to include hazardous drugs, controlled substances, oral liquids, and possibly powders and prefilled syringes. Our group purchasing organization (GPO) had an established agreement with an outsourced repackaging vendor. Knowing the GPO had inspected and vetted this repackager provided a certain comfort level. Nonetheless, we engaged the vendor on our own to learn more about their regulatory compliance, quality programs, supply chain workflow, staff training, and facilities, and we were satisfied with the results. The discounted pricing plan available through our GPO agreement was an additional incentive. We also confirmed that the vendor could create labeling similar to our current packaging; the goal is to ensure that the vendor’s labels are as similar as possible to in-house labeling in order to avoid confusion, particularly for nursing. At the same time, the vendor provided improvements over our in-house labeling as they offered additional elements, such as color-coding, a capability we did not have in-house.
Our evaluation of outsource repackaging demonstrated that the vendor was able to provide repackaged products at a lower cost and with improved quality over our current in-house process. Since repackaging is their main focus, our vendor has the equipment, experience, staff training, and familiarity with repackaging-specific regulatory requirements necessary to ensure a quality process.
Retaining In-house Capability
That said, we plan to maintain our in-house repackaging capability to fulfill certain needs, such as repackaging low-volume items on an as needed basis. For example, we may have a low-volume medication in bulk packaging with a two-year expiration. Rather than repackage it all at once and lose a year’s expiration on that product, we may repackage five or ten doses at a time as needed, to maintain the longer expiration date on the remaining product. In addition, with three 24-hour inpatient pharmacies, it is not unusual for each pharmacy to stock expensive but infrequently used drugs. To avoid waste from expired products, we now centralize these low-volume, high-dollar medications in the repackaging center, providing unit doses as needed.
Even as we transition much of our repackaging to an outside vendor, we still need to maintain some internal packaging operations. Our plan is to retain one FTE technician to oversee the ordering process, inventory, and database management for products that require repackaging. The technician will also continue to package about 400,000 low-volume doses annually. In addition, this technician will be tasked with reviewing our outsourcing quarterly in order to identify any products newly available in unit dose from the manufacturer, and to ensure we continue to receive competitive pricing on our outsourced products. We also will review par levels and make adjustments based on utilization.
Institutions need to weigh the options of the various repackaging models to determine the best combination given their distribution model and medication use technologies, while also balancing pricing against gains in efficiency. For those small community or rural hospitals servicing a streamlined patient population with a low repackaging volume, in-house repackaging may be the most cost-effective option. However, for facilities with a significant volume of repackaging, outsourcing can provide significant benefits. Consider whether repackaging is a task best managed in-house by your pharmacists and technicians, or is it more cost effective and efficient to outsource this responsibility, thus freeing your team for more patient-focused tasks.
 Christopher Fortier, PharmD, is the manager of pharmacy support and operating room services at the Medical University of South Carolina. He oversees procurement/contracting, three OR pharmacies, investigational drug, nuclear pharmacy, controlled substances, repackaging, and compounding services.
Christopher Fortier, PharmD, is the manager of pharmacy support and operating room services at the Medical University of South Carolina. He oversees procurement/contracting, three OR pharmacies, investigational drug, nuclear pharmacy, controlled substances, repackaging, and compounding services.
Managing Recalls
The process for managing recalls should also be considered for both in-house and outsourced repackaging. With in-house repackaging, a robust documentation system is necessary in order to locate any recalled medication, be it in pharmacy storage or already administered to the patient. We take a systematic approach using an electronic recall system internally, and our vendor also has a notification process where they provide us with the name of the recalled products, the lot number, and the shipment date. For our outsourced repackaged medications, this double alert makes it less likely that a recall will be overlooked.
Like what you've read? Please log in or create a free account to enjoy more of what www.pppmag.com has to offer.








