- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
- White Papers
New & Noteworthy
June 2024
Naloxone Hydrochloride Nasal Spray
Amneal Pharmaceuticals
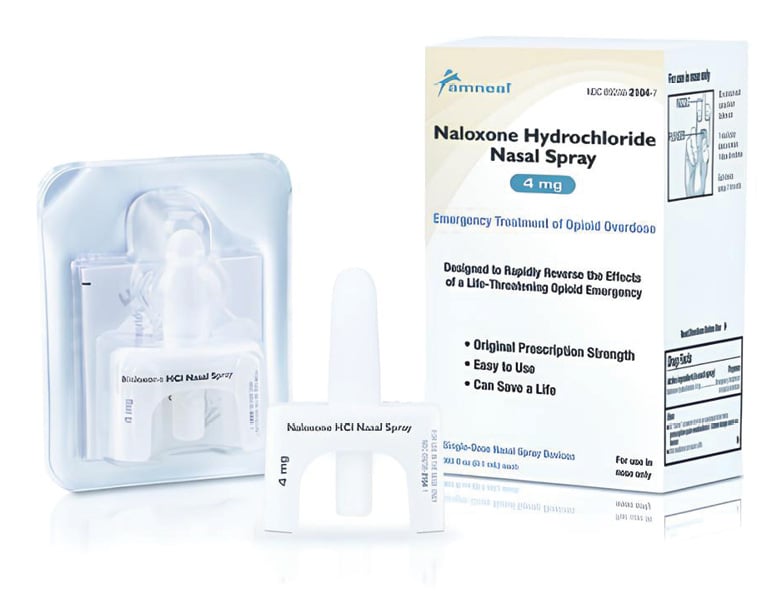
Amneal Pharmaceuticals announces the availability of over-the-counter naloxone hydrochloride (naloxone HCI) nasal spray, USP, 4 mg, following Abbreviated New Drug Application approval from the US FDA. The spray is manufactured in the US and is a generic equivalent to over-the-counter NARCAN HCI nasal spray, a medication that is widely used to help treat drug overdose from opioids, including heroin, fentanyl, and prescription opioid medications.
Recent Popular Articles
About Us
Pharmacy Purchasing & Products Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 PP&P Magazine - Pharmacy Purchasing & Products.
All rights reserved.